Main Menu
We’ve rebranded some of our products, learn more ›
CODEX® is now PhenoCycler,
Phenoptics™ is now Phenolmager.
Inviting you to join our User Group Meeting.
Spatial Biomarkers in Immuno-Oncology from Discovery to Translation.
Date: December 9, 2021, Thursday
Cancer immunotherapy achieves remarkable remissions in a subset of patients, but predictive markers are urgently needed to identify patients most likely to benefit. Multiplexed immunofluorescence (mIF) provides information not only about multiple markers on a cell, but spatial information about where different cells or cellular neighborhoods are located and how they interact to influence disease progression. Join us for this APAC user group meeting to learn how experts discover and establish the vital role of spatial biomarkers in setting the future directions in achieving precision Immuno-Oncology by using mIF technologies.
Talk 1: Single-Cell, Spatial Phenotyping: Fueling the Next Wave of Discovery Biology
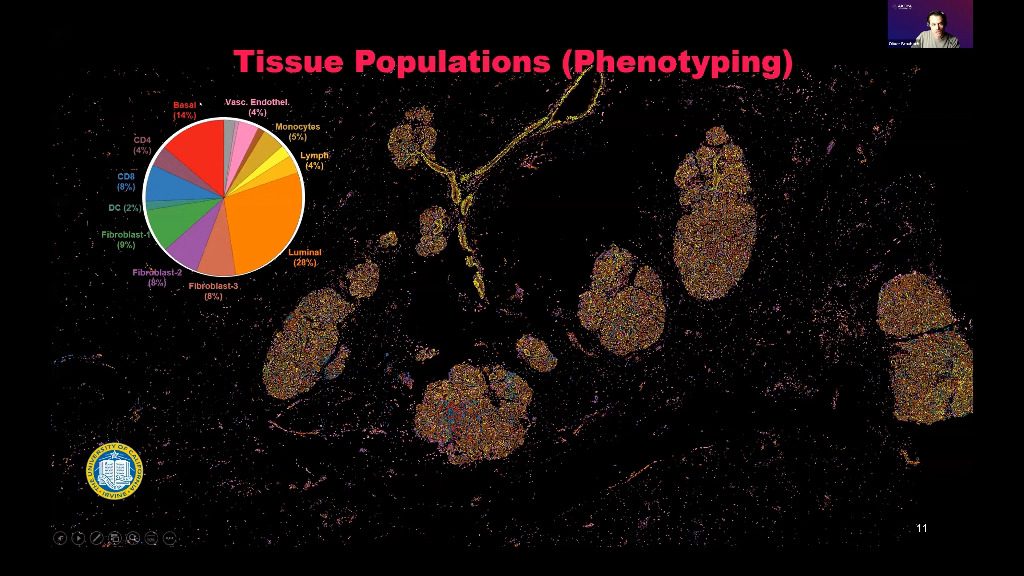
This talk explains multispectral immunofluorescence and multispectral imaging solutions in spatial biology, focus on the importance of single cell resolution in spatial biology, how to discover single cell contextual phenotypes and the many single-cell, spatial phenotyping applications.
Abstract
This talk explains multispectral immunofluorescence and multispectral imaging solutions in spatial biology, focus on the importance of single cell resolution in spatial biology, how to discover single cell contextual phenotypes and the many single-cell, spatial phenotyping applications.
Video
Speakers

Oliver Braubach, Ph.D.
Head of Research Applications, Akoya Biosciences
Talk 2: Understanding Tumor Immune Microenvironment using Multispectral Imaging
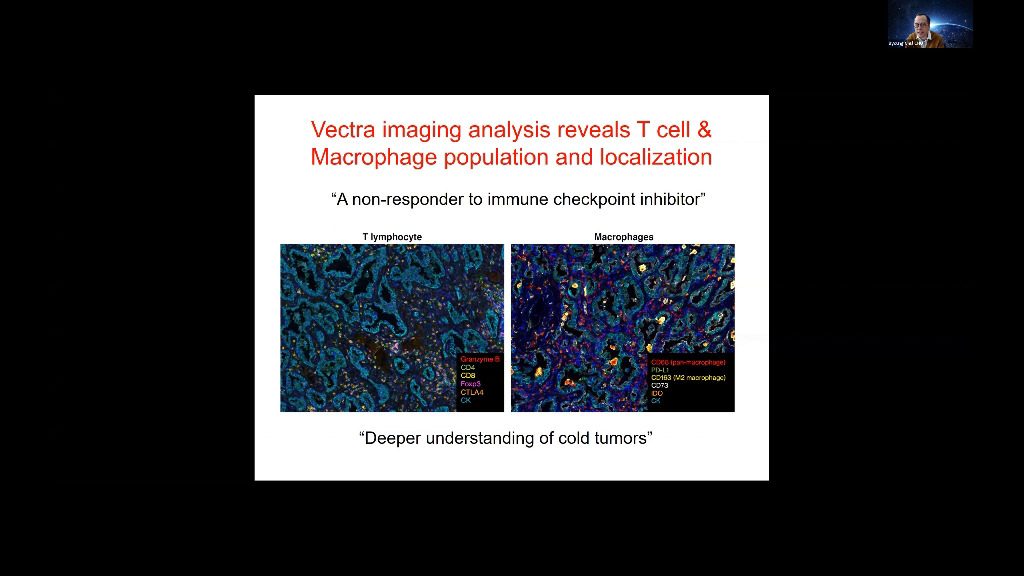
Tumors grow within an intricate network of epithelial cells, vascular and lymphatic vessels, cytokines and chemokines, and infiltrating immune cells. Different types of infiltrating immune cells have different effects on tumor progression, which can vary according to cancer type. Understanding immune microenvironment is crucial to develop effective cancer immunotherapies and identify resistance mechanisms to immunotherapy. A better visualization of and identification of cancer immunology biomarkers leads to improved understanding of the immune microenvironment. In my talk, I will describe Opal™ multiplex immunohistochemistry staining solutions, multispectral imaging systems, and advanced image-analysis software-, which enables a more comprehensive and specific view and analysis of the interaction between cancer and the immune system, at a cellular level, in a single tissue section.
Abstract
Tumors grow within an intricate network of epithelial cells, vascular and lymphatic vessels, cytokines and chemokines, and infiltrating immune cells. Different types of infiltrating immune cells have different effects on tumor progression, which can vary according to cancer type. Understanding immune microenvironment is crucial to develop effective cancer immunotherapies and identify resistance mechanisms to immunotherapy. A better visualization of and identification of cancer immunology biomarkers leads to improved understanding of the immune microenvironment. In my talk, I will describe Vectra Polaris- multiplex immunohistochemistry staining solutions, multispectral imaging systems, and advanced image-analysis software-, which enables a more comprehensive and specific view and analysis of the interaction between cancer and the immune system, at a cellular level, in a single tissue section.
Video
Speakers

Byoung Chul CHO, M.D., Ph.D.
Professor, the Division of Medical Oncology and Chief of the Lung Cancer Center at Yonsei Cancer Center, Yonsei University College of Medicine, South Korea
Talk 3: Spatial immunophenotypes in-depth discovery tumor immune microenvironment for prognostic and stratification of cancer patients
Characterizing immune phenotypes in human cancer tissues via multiplexed immunohistochemistry may help guide PD-L1 clinical therapy. The microenvironments analysis can improve our understanding of immune phenotypes and cell interactions and providing the ability to predict safe responses to immunotherapies.Demonstrated the need for the integration of multiplex IHC analysis to obtain more meaningful survival prognosis and patient stratification. Provides insights into the complex tumor immune evasion and immune regulation by the tumor-infiltrating effector and suppressor cells, informing on the best use of immunotherapy options.
Abstract
Characterizing immune phenotypes in human cancer tissues via multiplexed immunohistochemistry may help guide PD-L1 clinical therapy. The microenvironments analysis can improve our understanding of immune phenotypes and cell interactions and providing the ability to predict safe responses to immunotherapies.Demonstrated the need for the integration of multiplex IHC analysis to obtain more meaningful survival prognosis and patient stratification. Provides insights into the complex tumor immune evasion and immune regulation by the tumor-infiltrating effector and suppressor cells, informing on the best use of immunotherapy options.
Speakers

Dakang Xu, M.D., Ph.D.
Professor, Faculty of Medical Laboratory Science, Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University, China
Talk 4: Microenvironment of adult T-cell leukemia/lymphoma
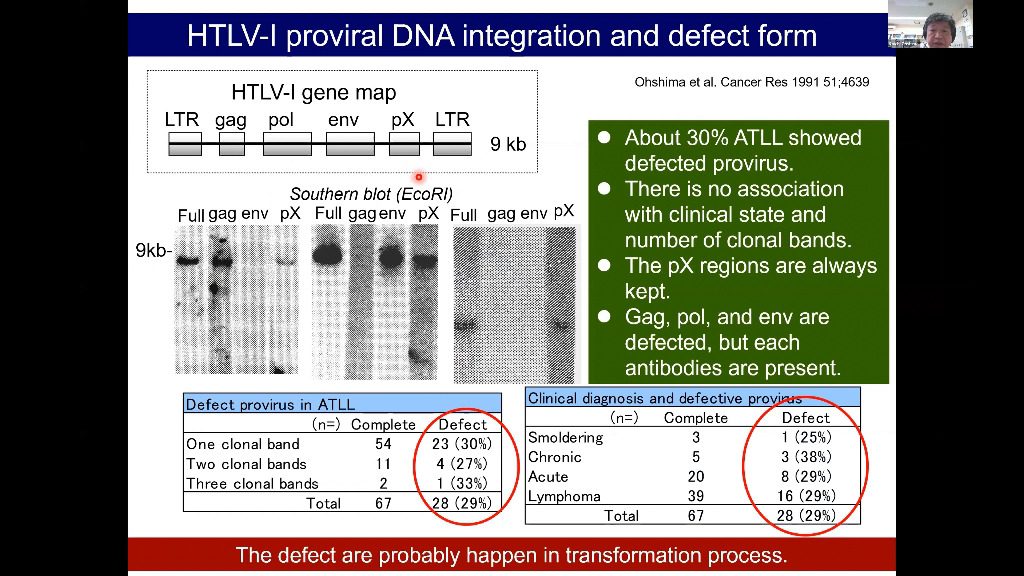
Adult T-cell leukemia/lymphoma (ATLL) is a malignancy caused by the human T-cell leukemia virus type 1. Aggressive ATLL is refractory to conventional chemotherapy and shows poor prognosis. The genetic landscape of ATLL reveals frequent genetic alterations in genes associated with immune surveillance, including major histocompatibility complex (MHC) class I and programmed cell death ligand 1. Clinicopathological investigations also reveal tumor immunity mechanisms in ATLL, including various immune checkpoint molecules, MHC molecules, tumor-associated macrophages, and chemokines. However, the tumor microenvironment of ATLL remains complex because ATLL itself is derived from T-cells, usually expressing regulatory T-cell markers. In this review, we discuss the recent literature describing the tumor microenvironment of ATLL.
Abstract
Adult T-cell leukemia/lymphoma (ATLL) is a malignancy caused by the human T-cell leukemia virus type 1. Aggressive ATLL is refractory to conventional chemotherapy and shows poor prognosis. The genetic landscape of ATLL reveals frequent genetic alterations in genes associated with immune surveillance, including major histocompatibility complex (MHC) class I and programmed cell death ligand 1. Clinicopathological investigations also reveal tumor immunity mechanisms in ATLL, including various immune checkpoint molecules, MHC molecules, tumor-associated macrophages, and chemokines. However, the tumor microenvironment of ATLL remains complex because ATLL itself is derived from T-cells, usually expressing regulatory T-cell markers. In this review, we discuss the recent literature describing the tumor microenvironment of ATLL.
Video
Speakers

Koichi Ohshima, M.D., Ph.D.
Professor of Pathology, School of Medicine, Kurume University, Japan
Talk 5: New dog, old tricks: “Oncofetal” reprogramming of the HCC tumor microenvironment promotes immune tolerance
Tumor cells exhibit phenotypic plasticity that contribute towards the generation of diverse cell states, thereby resulting in intra-tumor heterogeneity (ITH). ITH-driven diversity in the genetic and transcriptomic states within cancer cell populations are thought to be the major drivers of tumor evolution, both in the context of tumorigenesis and treatment-induced progression to resistant and/or metastatic disease. In my talk I will reflect upon our current understanding of how human liver cancer (HCC) might evolve in the context of their tumor microenvironment (TME). Specifically, I will share our recent observations from single cell transcriptomic studies on how cancer cells can reprogram the TME to generate a fetal-like immunosuppressive ecosystem in order to facilitate their growth and viability during carcinogenesis.
Abstract
Tumor cells exhibit phenotypic plasticity that contribute towards the generation of diverse cell states, thereby resulting in intra-tumor heterogeneity (ITH). ITH-driven diversity in the genetic and transcriptomic states within cancer cell populations are thought to be the major drivers of tumor evolution, both in the context of tumorigenesis and treatment-induced progression to resistant and/or metastatic disease. In my talk I will reflect upon our current understanding of how human liver cancer (HCC) might evolve in the context of their tumor microenvironment (TME). Specifically, I will share our recent observations from single cell transcriptomic studies on how cancer cells can reprogram the TME to generate a fetal-like immunosuppressive ecosystem in order to facilitate their growth and viability during carcinogenesis.
Speakers

Ramanuj DasGupta, Ph.D.
Senior Group Leader, Laboratory of Precision Oncology & Cancer Evolution, Spatial and Single Cell Domain, Genome Institute of Singapore, Singapore
Talk 6: Immune and stromal cells in the tumor microenvironment
Multiplexed immunofluorescence (mIF) imaging of tissue sections with the Akoya’s PhenoImager™ solution (formerly Phenoptics™) enables robust quantification of immune and stromal cell subsets with spatial context. mIF image analysis of tissue microenvironments also provides a comprehensive understanding of tumor biology and key insights for developing novel therapeutic strategies. In this presentation, we will describe how mIF and the PhenoImager workflow have been used for spatial profiling of different tumor microenvironments. Our data demonstrate unique cellular niches of multiple immune and stromal cell populations as well as their spatial interactions across whole tissue sections.
Abstract
Multiplexed immunofluorescence (mIF) imaging of tissue sections with the Akoya’s Phenoptics platform enables robust quantification of immune and stromal cell subsets with spatial context. mIF image analysis of tissue microenvironments also provides a comprehensive understanding of tumor biology and key insights for developing novel therapeutic strategies. In this presentation, we will describe how mIF and the Phenoptics platform have been used for spatial profiling of different tumor microenvironments. Our data demonstrate unique cellular niches of multiple immune and stromal cell populations as well as their spatial interactions across whole tissue sections.
Speakers

Saem Park, Ph.D.
Research fellow at the School of Biological Sciences, The University of Auckland, New Zealand
Talk 7: Multiplex imaging of Immunotherapy-treated melanoma
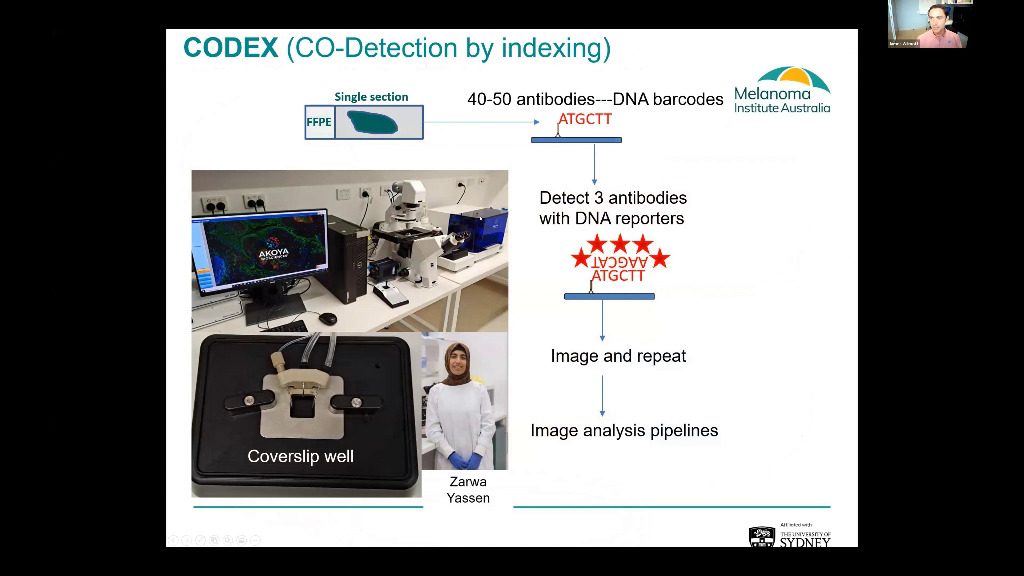
Approximately half of advanced melanoma patients respond to immune checkpoint based immunotherapies, yet 50% will still die due to resistance within 5 years. Therefore, it is critical that tools are developed which identify patients that are at high risk of drug resistance, and to find an alternate active therapy specific to their disease. Our research is focusing on a multi-omic approach, which includes multiplex immunofluorescence immune profiling of patient tumours to identify those unlikely to benefit from standard of care immunotherapies. Whilst using the highly multiplexed PhenoCycler™ (formerly CODEX®) workflow for staining of resistant tumours to understand the immunobiology of these tumours and to identify alternative treatment strategies. I will present the results of our ongoing feasibility trial which is using multispectral based imaging to predict the response of melanoma patients to standard of care, anti-PD-1+-anti-CTLA-4 immunotherapies. Along with our early exploration of immunotherapy resistant tumours using single cell RNA sequencing, combined with highly multiplex PhenoCycler tissue imaging.
Abstract
Approximately half of advanced melanoma patients respond to immune checkpoint based immunotherapies, yet 50% will still die due to resistance within 5 years. Therefore, it is critical that tools are developed which identify patients that are at high risk of drug resistance, and to find an alternate active therapy specific to their disease. Our research is focusing on a multi-omic approach, which includes multiplex immunofluorescence immune profiling of patient tumours to identify those unlikely to benefit from standard of care immunotherapies. Whilst using highly multiplex CODEX staining of resistant tumours to understand the immunobiology of these tumours and to identify alternative treatment strategies. I will present the results of our ongoing feasibility trial which is using multispectral based imaging to predict the response of melanoma patients to standard of care, anti-PD-1+-anti-CTLA-4 immunotherapies. Along with our early exploration of immunotherapy resistant tumours using single cell RNA sequencing, combined with highly multiplex CODEX tissue imaging.
Video
Speakers

James Wilmott, Ph.D.
NHMRC investigator fellow | Senior scientist at Melanoma Pathology and Translational Research Group, Melanoma Institute Australia, University of Sydney, Australia


